Feline Fever, Forever
Toxoplasma gondii is arguably one of the most interesting parasites known to nature. Packed in the single cell of this unassuming protozoan is the power to manipulate the most complex organ in the body – the brain- and remarkably, change the thoughts and behaviours of its unsuspecting host...
...by bringing on a case of the “cat crazy”, in mice and men.
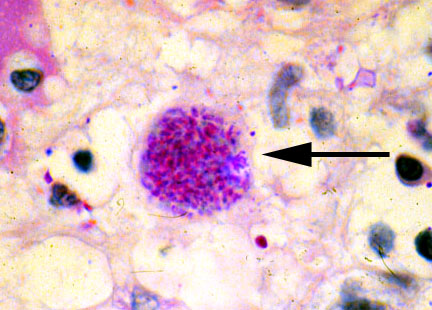
While T. Gondii can infect almost all warm-blooded animals, they are only able to sexually reproduce in the gut of our favourite feline – the cat. Once orally consumed, T. Gondii produces egg-like “off springs” in the gut of cats, which gets released into the wild with poop. There they sit until another potential host comes along – say, mice sniffling for crumbs or human changing the litter box – and once in, disseminates widely in the body and the brain, eventually forming cysts that are observable under the microscope.
T. Gondii cyst chilling in the brain. Can it really be harmless? Source: http://wiki.ggc.edu/
But now T. Gondii has a dilemma: to have a satisfying sexually active adult life (and continue the survival of the species), it HAS to get back into a cat. The best way to achieve this to get eaten. Astonishingly, T. Gondii evolved the ability to tweak its hosts’ brains and turn them into easily attainable catnip. Rodents, for example, loose their innate fear of cat urine, and may even become sexually aroused by cats.
While intriguing, previous behavioural studies mostly use the high-inflammatory Type II T. Gondii, which often leads to more infection-related complications and “sickness”-type behaviours in its host. This begs the question: is the parasite directly responsible for cat-love, or is the host’s inflammatory response that’s changing behaviour?
W.M. Ingram et al. (2013) Mice infected with low-virulence strains of Toxoplasma gondii lose their innate aversion to cat urine, even after extensive parasite clearance. PLOS ONE, doi:10.1371/journal.pone.0075246
The authors infected male mice with two low virulence strains (a genetically-altered Type I and a wild-type Type III) and waited between 3 weeks to 4 months for the parasite to “work its magic”. They then placed the mice into a box with cat urine on one end. The box was covered by a grid of infra-red beams; by tracking beam-breaks as the mice explored the box, researchers were able to closely track their position and movement.
As you can see in the graph below, uninfected mice avoided bobcat urine like the plague (solid red dots) while ignoring non-predatorial rabbit pee (empty red dots). The infected mice, on the other hand, didn’t think twice about approaching “dangerous” areas (solid triangle and solid square). This happened in an “all-or-non” manner regardless of how long they were infected, and didn’t disappear with time. A hidden cookie test revealed that their sense of smell was working fine.
When researchers looked for the presence of the parasite in the mice’s brain after the behavioral tests, they detected Type III T. Gondii DNA, but couldn’t find any signs of Type I.
Similarly, although Type III-infected mice had elevated white blood cells, suggesting an ongoing infection, Type I-infected had levels similar to those uninfected. Further probing confirmed that Type I did manage to established infection: parasite load was transiently detected between 5-20 days post-infection, along with molecular markers of a brain inflammatory response.
These results together suggest that (at least for Type I) T. Gondii can manipulate rodent behaviour even AFTER it’s gone and the brain no longer inflamed. Can the parasite still pull the cat-love strings 6 months after infection? What about a year? A lifetime?
T. Gondii thrives in 1/3 (!!!) of the human population, and once they settle, they stay for life. Once thought to be innocuous (other than the occasional“crazy cat lady” joke), troubling evidence is emerging that links T. Gondii infection to higher suicide risk, schizophrenia, depression and even Alzheimer’s disease in humans – without apparent signs of brain inflammation. Types I, II and III are the most prevalent types in North America, though I'd love to know how often humans get a low-virulent Type I infection as used here. Nevertheless, this study suggests that the “latent” or non-inflamed stage of T. Gondii may not be as asymptomatic as previously thought.
HOW is T. Gondii playing master puppeteer? Previously people have pointed the finger at inflammation and disruptive cysts. Increased levels of immunomodulatory molecules called cytokines is linked to depression and suicidal thoughts and hypothesized to mediate T. Gondii’s behavioural effects in humans. Cysts may directly increase dopamine production or disrupt neuronal activity in brain regions associated with mood and innate fear.
The finding that a transient infection can cause persistent changes suggest some other factors, such as synaptic plasticity, T. Gondii-inserted parasitic proteins, or even parasite-induced epigenetic changes may be involved.
So next time you change the litter box, remember to wear a pair of gloves and wash your hands after. Or risk succumbing to a prolonged –if not forever - case of the feline fever.
PS. T. Gondii infection is on the rise world wide – so's internet's love for all things cat-related. Correlation or causation? #catconspiracytheory
Wendy Marie Ingram, Leeanne M. Goodrich, Ellen A. Robey, & Michael B. Eisen (2013). Mice Infected with Low-Virulence Strains of Toxoplasma gondii Lose Their Innate Aversion to Cat Urine, Even after Extensive Parasite Clearance PLOS ONE DOI: 10.1371/journal.pone.0075246