Sap up the OCD
TL;DR: Using a mouse model of OCD, scientist use LIGHT to activate a brain area called the orbitofrontal cortex, which in turn shut down a downstream region called the striatum, and stopped OCD mouse from excessive face washing. Like magic.
Obsessive-compulsive disorder is a tough nut to crack. For years now researchers have been baffled by the spectrum of ritualistic and repetitive behaviours that relentlessly haunt those with the disorder. What’s more, intrusive thoughts and behaviours are not unique to OCD; they’re also prevalent in autism, drug abuse and eating disorders. We know that antidepressants help reduce symptoms, but only at high doses. As a first-line treatment, they’re still pretty pathetic.
Imaging studies from people with OCD tell us that a whole NETWORK of brain regions is disturbed in the disorder, including the orbitofrontal cortex and striatum; areas are associated with impulsivity and repetitive behaviours. However the data is strictly correlational, as we can’t tweak specific circuits in a targeted way in humans (although there are up-and-coming methods like deep brain stimulation and transcranial magnetic stimulation that zap an entire brain regions and modulate function). So what is the source DRIVING OCD behaviours and how can we stop it?
E Burguière et al. (2013). Optogenetic Stimulation of Lateral Orbitofronto-Striatal Pathway Suppresses Compulsive Behaviors. Science, 340 (6137):1243-1246
Seeking causative answers, researchers turned to mice. Specifically, a strain of anxious, compulsively over-grooming mice that lack a protein called SAPAP3. SAPAP3 is a protein at the synapse, the space between neurons where they communicate through neurotransmitter release.
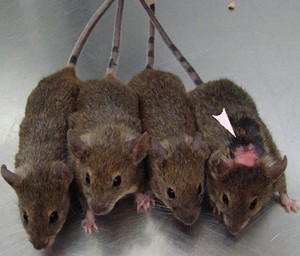
How to tell if a mouse has OCD-like traits? Look at it's grooming. Arrow points to an over-groomed mouse. Source:http://sfari.org/
When chilling in their home cages, mice like to occasionally groom their faces, which keeps them clean and happy. SAPAP3 knockout mice, however, take it to a whole different league. Given the chance –often in response to a “trigger”- they’ll groom so much that they tear their faces raw, not unlike OCD patients who compulsively scrub their hands until they bleed (not a pretty picture). Taking advantage of this trait, researchers first wanted to see if these mice would respond to a neutral trigger, as often happens in OCD patients. They played a tone, and then placed a drop of water on the mice, which drives them to groom it off. Soon, both normal and SAPAP3 knockout mice learned that a tune predicts water, and started grooming in response to the tune, as well as the water drop.
However, as time went on, normal mice (WT, black line) gradually stopped grooming at the tone – they learned that it really wasn’t necessary ("Late training", see how there's only a little bump in the black line after the tone/green line?). SAPAP3 knockouts (red line), on the other hand, couldn’t inhibit their grooming behaviour, relentlessly pawing to both the tune and the water drop ("Late training", see how there are two bumps in the red line,one after tone/green line and one after water/blue line?). This mimics the development of new OCD behaviours in patients, where a neutral trigger sets off a bout of repetitive actions.
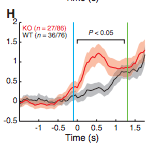
So what is happening at the neural level? Researchers focused on the orbitofrontal cortex (OFC) and striatum, areas associated with inhibiting responses and impulsivity, to see if the neurons fired differently. What they saw was, although firing rates of the OFC didn’t differ between normal and SAPAP3 knockouts, in the striatum, neuron firing rates were way higher in the SAPAP3 knockouts, especially as training went on. As you can see below, while normal mice (black line) “tuned” their striatal activity so that it only peaked near the time of the water drop (green line), SAPAP3 knockouts (black) had two peaks, one timed close to the tune (blue line) and one to the drop (green line). This is in line with their two grooming bouts – after the tune and the water drop.
Why this lack of inhibition? We know that activity in the OFC inhibits a type of neurons called the medium spiny neurons in the striatum, which in turn limits the degree of activation in this brain region. Could it be, then, that SAPAP3 knockout mice are missing this brake?
To answer this question, researchers brought in optogenetics, the technique that uses light to control neural activity. They used genetic methods to express a protein called channelrhodopsin-2 specifically in the OFC of SAPAP3 mice. They then used a light probe to “activate” this channel – that is, allow it to open so that the influx of ions allows neurons to fire. In this case, light on = OFC fires dramatically; light off = OFC little to no firing. Since connectivity in the brain is OFC->striatum, researchers recorded neural activity in the downstream striatum to see what happens when OFC activates.
What they found was that when OFC is activated by light (purple), the striatum goes quiet (see how the purple line is lower than the control black line?). That is, ACTIVATION of the OFC shuts down the striatum. How do we put this in the context of behavior? After the tone, when SAPAP3 knockouts usually have a spike in striatal activity (black line); if researchers activate OFC with light, this suppresses striatum activation. Is this enough to stop the animals from obsessively grooming to the tune?
Indeed it is! With the light on and the OFC activated, SAPAP3 knockouts behaved just like normal mice, staying chill during the tone, but grooming once they felt the annoying water drop. What’s more, light activation also decreased the high amount of face pawing that these mice usually exhibit while going about their usual daily micey-business. So all together, OFC activation can shut down the striatum, which reduces neurotic obsessive behaviour, but leaves normal behaviour intact.
This study obviously doesn’t provide an immediate cure for OCD, but it shines light (haha) on the circuits that go haywire in the disorder. It illuminates what we SHOULD be targeting when developing therapies in the future. This isn’t the end-all of OCD research; tons more questions remain. For example, which subtypes of medium spiny neurons are mediating this effect? When normal mice (and humans) develop OCD, which brain area goes awry first? What are the molecular mechanisms behind the lack of inhibition seen in the striatum of OCD-like mice? Can the same manipulation treat OCD-like behaviours in other psychopathological models, such as autism and eating disorders?
A little more on the last question. For a long time now physicians have noticed a link between OCD-like behaviours and eating disorders, such as anorexia nervosa. A recent paper made the first step in discovering a potential molecular link between the two disorders. Using the same SAPAP3 knockout mice, these researchers found that if you knockout a gene called MC4R at the same time (or inhibit the protein using drugs), you can alleviate compulsive grooming seen in these mice.
What’s even more interesting, although MC4R knockouts are usually obese (left, MC4R-null), if you eliminate both MC4R and SAPAP3, you get a normal, non-obsessive slim mouse (right, double null). In this case, two wrongs made a right. Researchers think that this MC4R-SAPAP3 link may explain to some degree the co-occurance of OCD-like behaviors and maladaptive food intake. Scicurious has a great write-up of this study if you’d like to know more.
Burguière E, Monteiro P, Feng G, & Graybiel AM (2013). Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science (New York, N.Y.), 340 (6137), 1243-6 PMID: 23744950